Table of Contents
Understanding Salinity: The Measure of Dissolved Salts in Water
Salinity is a term used to describe the measure of the amount of dissolved salts in water. It is usually expressed in grams of salt per liter or kilogram of water, or in parts per thousand (‰). Salinity affects numerous physical and chemical properties of water, such as its density, heat capacity, conductivity, and freezing point. Furthermore, salinity plays a crucial role in determining the types of organisms that can thrive in different aquatic environments.
Source of Oceanic Salinity
The primary source of oceanic salinity is the land. Rivers carry salts in solution form from the continental areas, contributing to the salinity of the oceans. Additionally, volcanic ashes are a major source of oceanic salinity.
Controlling Factors of Salinity
Several factors influence the salinity levels in different oceans and seas. These factors are referred to as controlling factors of oceanic salinity:
- Evaporation: There is a direct positive relationship between the rate of evaporation and salinity. Higher evaporation rates, resulting from high temperatures and low humidity, cause increased salt concentration and higher overall salinity. For example, the salinity is generally higher near the tropics due to the high rate of evaporation in dry air over the tropics of Cancer and Capricorn.
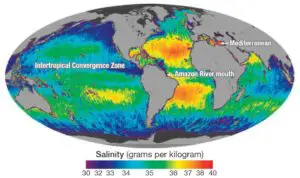
- Precipitation: Precipitation is inversely proportional to salinity. Regions with high rainfall, such as the equatorial region, tend to have lower salinity compared to areas with low rainfall, such as the subtropical high-pressure belt.
- Influx of river water: Rivers bring salt from the land to the oceans. However, large rivers pouring immense amounts of water into the ocean can reduce salinity at their mouths. On the other hand, regions with higher rates of evaporation than influx of fresh river water experience an increase in salinity.
- Atmospheric pressure and wind direction: Stable air with high temperatures caused by anti-cyclonic conditions can increase the salinity of surface ocean water. Prevailing winds help redistribute salt in the oceans and seas, resulting in changes in salinity levels. For example, westerlies increase salinity along the western coasts of continents while lowering salinity along the eastern coasts.
- Circulation of oceanic water: Oceanic currents play a role in mixing seawaters and affecting the spatial distribution of salinity. Equatorial warm currents drive away salts from western coastal areas of continents and accumulate them along eastern coastal areas, influencing the salinity levels in those regions.
Composition of Seawater
Seawater contains a composite solution of various mineral substances in dilute form due to its role as an active solvent. The composition of seawater includes:
| Salts | Amount (‰) | Percentage |
|---|---|---|
| Sodium chloride (NaCl) | 27.2 | 77.8% |
| Magnesium chloride (MgCl2) | 3.8 | 10.9% |
| Magnesium sulfate (MgSO4) | 1.6 | 4.7% |
| Calcium sulfate (CaSO4) | 1.2 | 3.6% |
| Potassium sulfate (K2SO4) | 0.8 | 2.5% |
| Calcium carbonate (CaCO3) | 0.1 | 0.3% |
| Magnesium bromide (MgBr2) | 0.1 | 0.2% |
Distribution of Salinity
Salinity distribution in oceans and seas can be analyzed from both horizontal and vertical perspectives.
Horizontal Distribution of Salinity
The latitudinal distribution of salinity shows a decreasing trend from the equator towards the poles, with the highest salinity typically recorded around 20-40 degrees north latitude. However, the equatorial zone itself accounts for a relatively low salinity of approximately 35‰. The northern and southern hemispheres, on average, record salinity levels of 31‰ and 34‰, respectively. Marginal areas of oceans bordering continents generally have lower salinity due to the influx of fresh river waters.
Vertical Distribution of Salinity
The vertical distribution of salinity in the ocean varies with depth. In general, salinity levels decrease with depth, as surface waters tend to be influenced by factors such as evaporation and precipitation, while deeper waters are less affected by these processes. Vertical distribution plays a significant role in oceanographic studies, as it can provide insights into the mixing and circulation of oceanic waters.
Significance of Salinity
Salinity is of great importance in various fields, including oceanography, climate science, and marine biology. It helps scientists understand the composition and behavior of seawater, influences ocean currents and climate patterns, and affects the distribution and survival of marine organisms. The study of salinity provides valuable information for environmental monitoring, marine resource management, and predicting the impacts of climate change on aquatic ecosystems.
Fun Fact: Why Man is Seldom Drowned in Sea Water with Very High Salinity?
The high salinity of sea water makes it denser than the human body, resulting in increased buoyancy. This buoyancy makes it easier for humans to float and stay afloat in sea water with high salinity, reducing the likelihood of drowning. In comparison, freshwater bodies have lower salinity and lower buoyancy, making it more difficult to float and increasing the risk of drowning.
In conclusion, salinity is a crucial property of water, influencing various aspects of aquatic environments. Understanding the factors and distribution of salinity contributes to our knowledge of oceanography, climate science, and marine ecosystems.
Mutiple Choice Questions
1. What is salinity?
A) The weight of sea water
B) The weight of dissolved materials in sea water
C) The weight of salt per liter of water
D) The weight of salt per kilogram of water
Explanation: Salinity is defined as the ratio between the weight of dissolved materials and the weight of the sample sea water.
2. How is salinity usually expressed?
A) Grams of salt per liter of water
B) Grams of salt per kilogram of water
C) Parts per thousand (‰)
D) All of the above
Explanation: Salinity is usually expressed in grams of salt per liter or kilogram of water, or in parts per thousand (‰).
3. How does salinity affect water?
A) It increases the density of water
B) It influences the freezing point of water
C) It affects the heat capacity of water
D) All of the above
Explanation: Salinity affects many physical and chemical properties of water, such as its density, heat capacity, conductivity, and freezing point.
4. What is the primary source of oceanic salinity?
A) Volcanic ashes
B) Evaporation
C) Influx of river water
D) Rainfall
Explanation: The primary source of oceanic salinity is land, as rivers bring salts in solution form from the continental areas. Volcanic ashes are also a major source of oceanic salinity.
5. How does evaporation affect salinity?
A) It increases salinity
B) It decreases salinity
C) It has no effect on salinity
D) It depends on other factors
Explanation: Evaporation has a direct positive relationship with salinity. Higher rates of evaporation result in higher salinity, and vice versa.
6. How does precipitation affect salinity?
A) It increases salinity
B) It decreases salinity
C) It has no effect on salinity
D) It depends on other factors
Explanation: Precipitation is inversely proportional to salinity. Higher rainfall leads to lower salinity, and vice versa.
7. How does the influx of river water affect salinity?
A) It increases salinity
B) It decreases salinity
C) It has no effect on salinity
D) It depends on other factors
Explanation: The influx of river water reduces salinity at the mouth of the ocean. Where evaporation exceeds the influx of fresh river waters, there is an increase in salinity.
8. How do atmospheric pressure and wind direction affect salinity?
A) They increase salinity
B) They decrease salinity
C) They have no effect on salinity
D) It depends on other factors
Explanation: Anti-cyclonic conditions with stable air and high temperature increase the salinity of surface water. Winds also help redistribute salt in the oceans and seas.
9. How do oceanic currents affect salinity?
A) They increase salinity
B) They decrease salinity
C) They have no effect on salinity
D) It depends on other factors
Explanation: Oceanic currents affect the spatial distribution of salinity by mixing seawaters. Warm currents drive salts away from western coastal areas and accumulate them along eastern coastal areas.
10. What is the composition of seawater?
A) Mostly sodium chloride (NaCl)
B) Mostly magnesium chloride (MgCl2)
C) Mostly calcium sulfate (CaSO4)
D) All of the above
Explanation: Seawater contains a composite solution of many mineral substances. The most abundant is sodium chloride (NaCl), followed by magnesium chloride (MgCl2), magnesium sulfate (MgSO4), calcium sulfate (CaSO4), and other minerals.
Brief Summary | UPSC – IAS
Salinity is the measure of the amount of dissolved salts in water and affects many physical and chemical properties of water. The primary source of oceanic salinity is land, with rivers bringing salts into the oceans. The factors that affect the amount of salinity in different oceans and seas include evaporation, precipitation, the influx of river water, prevailing winds, ocean currents, and sea waves. Different factors can increase or decrease salinity in certain areas. For example, evaporation leads to higher salinity, while precipitation leads to lower salinity. The spatial distribution of salinity varies horizontally and vertically, with latitudinal distribution showing decreasing salinity from the equator towards the poles. The regional distribution of salinity varies in individual oceans and seas.