Table of Contents
Understanding Salinity: The Measure of Salt in Water
Salinity is a crucial aspect of our oceans and seas, influencing various physical and chemical properties of water. It refers to the measure of the amount of dissolved salts in water and is typically expressed in grams of salt per liter or kilogram of water, or in parts per thousand (‰).
Source of Oceanic Salinity
The primary source of oceanic salinity is the land, with rivers bringing salts in solution form from continental areas. Additionally, volcanic ashes also contribute significantly to oceanic salinity. However, the salts brought by rivers undergo some modifications in the oceans, making volcanic ashes a major source of salinity in the oceans.
Controlling Factors of Salinity
Several factors affect the amount of salinity in different oceans and seas. These factors are known as controlling factors of oceanic salinity and include:
- Evaporation: There is a direct positive relationship between the rate of evaporation and salinity. Higher temperatures and low humidity cause more concentration of salt, resulting in higher salinity.
- Precipitation: Precipitation is inversely proportional to salinity. Higher rainfall leads to lower salinity, while lower rainfall results in higher salinity.
- Influx of river water: Rivers bring salt from the land to the oceans, reducing salinity at their mouths. However, when evaporation exceeds the influx of fresh river waters, salinity increases.
- Atmospheric pressure and wind direction: Anti-cyclonic conditions with stable air and high temperature increase salinity. Wind direction helps redistribute salt in the oceans, causing variations in salinity along the coasts.
- Circulation of oceanic water: Oceanic currents mix seawaters and play a role in the spatial distribution of salinity. Equatorial warm currents drive away salts from the western coastal areas of continents and accumulate them along the eastern coastal areas.
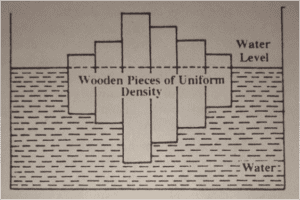
Why Man is Seldom Drowned in High Salinity Sea Water?
The reason why humans are seldom drowned in sea water with very high salinity is due to the principle of buoyancy. Saltwater is denser than freshwater, allowing humans to float more easily. The high salinity of the water provides greater buoyancy, making it easier for individuals to stay afloat. Additionally, the high amount of dissolved salts affects the physical properties of water, increasing its density and providing additional support for buoyancy. However, prolonged exposure to highly salty water can be harmful to the human body due to dehydration and other health issues.
Significance of Salinity
Salinity has significant implications for aquatic life and the functioning of marine ecosystems. It determines the types of organisms that can thrive in different aquatic environments. Some organisms, such as certain types of fish and marine plants, have adapted to high salinity environments, while others are better suited for low salinity areas. Salinity also affects the water’s freezing point, heat capacity, conductivity, and density. Understanding salinity is crucial for studying and managing marine ecosystems, as well as for various industries that rely on oceanic resources.
Fun Fact:
The Dead Sea, located between Jordan and Israel, is one of the saltiest bodies of water on Earth. Its salinity is approximately 34.2%, almost ten times saltier than the regular ocean water. Due to its high salt concentration, it is effortless for individuals to float on the surface without sinking.
Mutiple Choice Questions
1. What is salinity?
a. The weight of the dissolved materials in sea water
b. The weight of the sample sea water
c. The amount of dissolved salts in water
d. The ratio between the weight of the dissolved materials and the weight of sample sea water
Explanation: Salinity is defined as the ratio between the weight of the dissolved materials and the weight of sample sea water.
2. How is salinity usually expressed?
a. In grams of salt per liter or kilogram of water
b. In grams of salt per milliliter of water
c. In parts per million (ppm)
d. In parts per thousand (‰)
Explanation: Salinity is usually expressed in grams of salt per liter or kilogram of water, or in parts per thousand (‰).
3. How does salinity affect water?
a. It affects its density, heat capacity, conductivity, and freezing point
b. It affects its color, odor, and taste
c. It changes its pH level
d. It has no effect on water
Explanation: Salinity affects many physical and chemical properties of water, such as its density, heat capacity, conductivity, and freezing point.
4. What is the primary source of oceanic salinity?
a. Volcanic ashes
b. Evaporation
c. Precipitation
d. Rivers
Explanation: The primary source of oceanic salinity is land, as rivers bring salts in solution form from the continental areas.
5. What is the relationship between evaporation and salinity?
a. There is a direct positive relationship between the rate of evaporation and salinity
b. There is a direct negative relationship between the rate of evaporation and salinity
c. There is an inverse relationship between the rate of evaporation and salinity
d. There is no relationship between evaporation and salinity
Explanation: There is a direct positive relationship between the rate of evaporation and salinity, meaning that greater the salinity, higher the evaporation rate.
6. How does precipitation affect salinity?
a. It increases salinity
b. It decreases salinity
c. It has no effect on salinity
d. It depends on the amount of precipitation
Explanation: Precipitation is inversely proportional to salinity, meaning that higher the rainfall, lower the salinity, and vice versa.
7. How does the influx of river water affect salinity?
a. It increases salinity
b. It decreases salinity
c. It has no effect on salinity
d. It depends on the river
Explanation: The influx of river water reduces the salinity at the mouth of the ocean, as the large volume of fresh water dilutes the salt concentration.
8. How do atmospheric pressure and wind direction affect salinity?
a. They increase salinity
b. They decrease salinity
c. They have no effect on salinity
d. It depends on the specific conditions
Explanation: Anti-cyclonic conditions with stable air and high temperature increase the salinity of the surface water of the oceans. Winds also help in redistributing salt in the oceans and seas, resulting in changes in salinity.
9. What is the composition of seawater?
a. A mixture of various gases
b. A composite solution of mineral substances in a dilute form
c. A mixture of organic compounds
d. Pure water without any dissolved substances
Explanation: Seawater contains a composite solution of a huge amount of mineral substances in a dilute form, making it an active solvent.
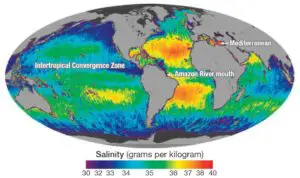
10. How does salinity vary with latitudinal distribution?
a. Salinity increases from the equator towards the poles
b. Salinity decreases from the equator towards the poles
c. Salinity is the highest near the equator
d. Salinity remains constant throughout the latitudes
Explanation: On an average, salinity decreases from the equator towards the poles, with the highest salinity recorded at 20-40 degrees north latitude.
Brief Summary | UPSC – IAS
Salinity is the measure of the amount of dissolved salts in water. It affects the physical and chemical properties of water and determines the types of organisms that can live in different aquatic environments. The primary source of oceanic salinity is from land, where rivers bring salts in solution form. The factors that affect salinity include evaporation, precipitation, influx of river water, prevailing winds, ocean currents, and sea waves. Evaporation increases salinity, while precipitation decreases it. The composition of seawater contains a wide variety of mineral substances in dilute form. Salinity varies from enclosed seas to open seas, with higher salinity in tropical zones and lower salinity near the equator and poles. Marginal areas of oceans have lower salinity due to the influx of fresh water from rivers.