Table of Contents
Understanding Salinity: A Comprehensive Guide
Salinity is a crucial concept in the field of oceanography and environmental studies. It refers to the measure of the amount of dissolved salts in water and is usually expressed in grams of salt per liter or kilogram of water, or in parts per thousand (‰). Salinity plays a significant role in shaping the physical and chemical properties of water, such as density, heat capacity, conductivity, and freezing point, as well as influencing the types of organisms that can thrive in different aquatic environments.
Source of Oceanic Salinity
The primary source of oceanic salinity is the land. When rivers flow into the oceans, they bring salts in solution form from the continental areas. Additionally, volcanic ashes also contribute to the salinity of oceans. However, the salts brought by rivers undergo modification in the oceans before contributing to the overall salinity.
Controlling Factors of Salinity
Several factors influence the salinity of different oceans and seas and are therefore referred to as controlling factors of oceanic salinity. These factors include evaporation, precipitation, influx of river water, prevailing winds, ocean currents, and sea waves.
Evaporation
Evaporation has a direct positive relationship with salinity. Higher rates of evaporation lead to higher salinity, and vice versa. When evaporation occurs due to high temperatures and low humidity (dry conditions), it causes a higher concentration of salt, resulting in increased salinity. The salinity is higher near the tropics due to the high rate of evaporation combined with dry air over the tropics of Cancer and Capricorn.
According to Wust (1935), the mean annual rate of evaporation in the Atlantic Ocean is 94cm at 40 degrees north latitude, 149cm at 20 degrees north latitude, and 105cm near the equator. Salinity is 34.68‰ at 5 degrees north and more than 37‰ at 20 degrees north. In the southern Atlantic Ocean, evaporation is 143cm/year at 10 degrees south. Sub-tropical pressure belts and trade wind belts, which experience rapid evaporation, have higher salinity.
Precipitation
Precipitation is inversely proportional to salinity. Higher rainfall results in lower salinity, and vice versa. Regions with high rainfall, such as equatorial regions, record lower salinity compared to regions with low rainfall, like the subtropical high-pressure belt.
Influx of River Water
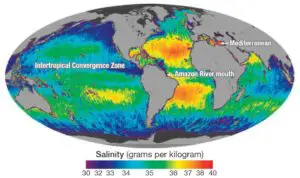
Rivers bring salt from the land to the oceans. However, rivers that pour down immense amounts of water at the mouth of the ocean reduce the salinity at that point. For example, the mouth of the Ganges, Congo, Niger, and Amazon rivers all contribute to reduced salinity. Salinity increases when evaporation surpasses the influx of fresh river water. The Mediterranean Sea, for instance, records a salinity of 40‰.
Atmospheric Pressure and Wind Direction
Anti-cyclonic conditions with stable air and high temperatures increase the salinity of ocean surface water. Sub-tropical high-pressure belts exhibit such conditions and cause high salinity. Winds also play a role in redistributing salt in the oceans and seas. They drive away saline water to less saline areas, resulting in a decrease in salinity in the former and an increase in the latter. Westerly winds increase salinity along the western coasts of continents but lower salinity along the eastern coast.
Why Does High Salinity in the Sea Water Not Cause Drowning?
Despite the high salinity in sea water, individuals are unlikely to drown due to it. This is because the human body contains a lower concentration of salts compared to the seawater. When immersed in highly saline water, osmosis occurs, causing water to move from areas of low salt concentration (the body) to areas of high salt concentration (the surrounding water). This movement of water prevents the body from absorbing excessive amounts of salt, making drowning unlikely in very high salinity seawater.
Composition of Seawater
Seawater contains a composite solution of various mineral substances in dilute form because it is an active solvent. The composition of seawater includes salts such as sodium chloride (27.21‰), magnesium chloride (3.80‰), magnesium sulfate (1.65‰), calcium sulfate (1.26‰), potassium sulfate (0.86‰), calcium carbonate (0.12‰), and magnesium bromide (0.08‰). These minerals contribute to the overall salinity of seawater.
Distribution of Salinity
The distribution of salinity in oceans and seas is studied in terms of both horizontal and vertical distribution.
Horizontal Distribution of Salinity
Salinity decreases on average from the equator towards the poles, although the highest salinity is seldom recorded near the equator. The equator accounts for only 35‰ of salinity, while the highest salinity (around 36‰) is recorded at 20-40 degrees north latitude. The average salinity between 10-30 degrees south latitude is 35‰. Low salinity is observed at 40-60 degrees in both hemispheres. On average, the northern hemisphere records an average salinity of 31‰, while the southern hemisphere records an average salinity of 34‰.
The salinity of marginal areas of oceans bordering continents is lower than their central parts due to the addition of freshwaters from rivers. However, the salinity of partially enclosed seas in higher latitudes is influenced by factors other than latitude, such as influx of meltwater. For example, the Baltic Sea has lower salinity than the North Sea, despite both having the same latitudinal extent.
Regional Distribution of Salinity
In terms of regional distribution, Jenkins divided the oceans into three categories based on salinity variations:
1. Seas with salinity above the norm:
- Red Sea (34-41‰)
- Persian Gulf (37-38‰)
- Mediterranean Sea (37-39‰)
2. Seas with normal salinity:
- Caribbean Sea and Gulf of Mexico (35-36‰)
- Bass Strait (35‰)
- Gulf of California (25-35‰)
3. Seas with salinity below the norm:
- Arctic Sea, North Australian Sea, Bering Sea, Okhotsk Sea, Japan Sea, China Sea, Andaman Sea, North Sea, English Channel, Gulf of St. Lawrence (slightly less)
- Baltic Sea, Hudson Bay (much below)
Overall, understanding salinity is essential in comprehending the complex dynamics of our oceans and the diverse ecosystems they support. It helps scientists and researchers monitor changes in salinity patterns, which can have implications for both marine life and climate.
Fun Fact: Did you know that the Dead Sea, located between Jordan and Israel, has one of the highest salinity levels in the world? With a salinity level of around 342‰, it is almost 10 times saltier than the average ocean!
Mutiple Choice Questions
1. What is salinity?
a) The amount of dissolved salts in water
b) The weight of dissolved materials in sea water
c) The measure of the weight of sample sea water
d) The ratio of weight of dissolved materials to weight of sea water
Explanation: Salinity is the measure of the amount of dissolved salts in water. It is usually expressed in grams of salt per liter or kilogram of water, or in parts per thousand (‰).
2. What are the controlling factors of salinity?
a) Evaporation, precipitation, influx of river water, and ocean currents and sea waves
b) Influx of river water, atmospheric pressure, wind direction, and evaporation
c) Ocean currents and sea waves, evaporation, precipitation, and composition of seawater
d) Precipitation, wind direction, composition of seawater, and atmospheric pressure
Explanation: The factors that affect the salinity of different oceans and seas are known as controlling factors of oceanic salinity. These factors include evaporation, precipitation, influx of river water, prevailing winds, ocean currents, and sea waves.
3. What is the relationship between evaporation and salinity?
a) Direct positive relationship
b) Direct negative relationship
c) Inverse positive relationship
d) Inverse negative relationship
Explanation: There is a direct positive relationship between the rate of evaporation and salinity. This means that greater the rate of evaporation, the higher the salinity, and vice versa. Evaporation leads to higher salinity due to the concentration of salt in water.
4. How does precipitation affect salinity?
a) It has no effect on salinity
b) It decreases salinity
c) It increases salinity
d) It depends on the amount of precipitation
Explanation: Precipitation is inversely proportional to salinity, meaning that higher rainfall leads to lower salinity and vice versa. Regions with high rainfall, such as equatorial regions, tend to have lower salinity compared to regions with low rainfall, like subtropical high-pressure belts.
5. What is the primary source of oceanic salinity?
a) Volcanic ashes
b) Rivers
c) Evaporation
d) Sea waves
Explanation: The primary source of oceanic salinity is land. Rivers bring salts in solution form from the continental areas into the oceans. Volcanic ashes are also a significant source of oceanic salinity.
6. How do ocean currents affect the distribution of salinity?
a) They have no effect on salinity
b) They increase salinity in all areas
c) They decrease salinity along western coasts and increase it along eastern coasts
d) They decrease salinity along eastern coasts and increase it along western coasts
Explanation: Ocean currents affect the spatial distribution of salinity by mixing seawaters. Warm equatorial currents drive away salts from western coastal areas and accumulate them along eastern coastal areas, leading to higher salinity. Currents have the least influence on enclosed and marginal seas.
7. What is the composition of seawater?
a) Only sodium chloride (NaCl)
b) A composite solution of various mineral substances
c) Mainly magnesium chloride (MgCl2) and magnesium sulphate (MgSO4)
d) Mostly calcium sulphate (CaSO4) and calcium carbonate (CaCO3)
Explanation: Seawater contains a composite solution of various mineral substances in a dilute form. The major salts in seawater include sodium chloride (NaCl), magnesium chloride (MgCl2), magnesium sulphate (MgSO4), calcium sulphate (CaSO4), potassium sulphate (K2SO4), calcium carbonate (CaCO3), and magnesium bromide (MgBr2).
8. How does salinity vary with latitude?
a) Salinity decreases from the equator towards the poles
b) Salinity increases from the equator towards the poles
c) Salinity is highest near the equator and lowest at the poles
d) Salinity is constant at all latitudes
Explanation: Salinity generally decreases from the equator towards the poles. However, higher salinity is seldom recorded near the equator. The highest salinity is usually found at 20-40 degrees north latitude, while low salinity is observed at 40-60 degrees in both hemispheres.
9. Which oceans have salinity above the normal range?
a) Atlantic Ocean, Indian Ocean, and Arctic Ocean
b) Red Sea, Persian Gulf, and Mediterranean Sea
c) North Pacific Ocean, South Pacific Ocean, and Southern Ocean
d) Caribbean Sea, Gulf of Mexico, and Gulf of California
Explanation: The Red Sea, Persian Gulf, and Mediterranean Sea have salinity above the normal range. Red Sea has a salinity range of 34-41 0/00, Persian Gulf has 37-38 0/00, and Mediterranean Sea has 37-39 0/00.
10. Which factor mainly controls the salinity of partially enclosed seas in higher latitudes?
a) Latitude
b) Melt-water influx
c) Ocean currents
d) Evaporation
Explanation: The salinity of partially enclosed seas in higher latitudes is mainly controlled by the influx of melt-water. This means that the amount of freshwater entering these seas from melting ice and snow dictates their salinity levels.
Brief Summary | UPSC – IAS
Salinity is the measure of the amount of dissolved salts in water and affects various properties of water and the types of organisms that can live in it. The primary source of oceanic salinity is the land, as rivers bring salts from the continental areas. The factors that control salinity include evaporation, precipitation, influx of river water, prevailing winds, ocean currents, and sea waves. Evaporation and precipitation have an inverse relationship with salinity, while the influx of river water reduces salinity at the mouth of rivers. Atmospheric pressure, wind direction, and oceanic currents also affect salinity. Salinity varies horizontally with latitude and regionally within individual oceans.