Table of Contents
Distribution of Salinity, Density and Temperature of Sea Water | UPSC
The world ocean meaning the combined oceans of the earth, occupying about 71% of the earth’s surface & has a mean depth of about 3800m including shallow seas in addition to the main basins. The round figure of 4000m applies quite well to the average depth of the main portions of the Atlantic, Pacific 7 Indian Oceans. Volume of the world ocean is about 1.4 billion cubic kilometers (1.37×10 to the power of 9 cu km) which constitutes 97.2 % of the world’s free water most of the remaining 2.8% is locked up in glaciers.
Physio-chemical characteristics of seawater
Physical characteristics of sea water-
- Water is the sole natural compound which exists in 3 statuses in the conditions of temperature & pressure which are found on earth. The liquid state being the most common.
- Water has high specific heat, linked on the one hand, to the fact that one its constituents hydrogen has the highest specific heat of all the elements & on the other hand to the presence of hydrogen bonds.
- The freezing of water is accompanied by an increase in volume of about 105 & because of this ice floats on water, freezing splits the partitions of cells in animal or plant & porous.
- The surface tension of water is the highest of all liquids. This characteristic influences the formation of drops of water as well as waves.
- The surface water in a liquid state reflects only a small part of luminous radiation & absorbs much solar heat.
- The transmission of light is unaffected by salinity temperature or pressure. However suspended particle may scatter the light. Is more penetrating & they are subjected to molecular scattering & hence the blue color of the ocean. Different colors of the ocean like green or brownish particularly along coast due to green planktonic species & due to detritus suspended the water.
- Sound waves propagation is easily affected by factors like salinity, temperature or pressure. The speed of waves increases with increase in salinity, temperature or pressure.
The chemical composition of sea-water
Ocean water contains variety of substances dissolved in water & as suspended particles. The composition of sea water vary from place to place 7 is primarily depends on the abundance of life forms, presence of rivers & other geological & meteorological conditions. Thus different substances are dissolved in sea water. The dissolved substances of seawater is therefore can be into 2 categories namely
Dissolved gases in sea-water
- The major gases found in sea water in the order of their relative abundances are Nitrogen, Oxygen & Carbon dioxide. Apart from these the presence of hydrogen sulphide gas is significant as it indicate bacterial activity, decay of organic material & stagnation of water.
The mineral constituents of water
- The sea contains a large number of dissolved compounds & elements. The seawater contains about 10 major elements & at least about 49 minor & trace elements.
The ten major elements of the sea water & their concentrations are listed below.
| Elements | Cl | Na | Mg | S | Ca | K | Br | C | Sr | B |
| % | 19.35 | 10.79 | 1.29 | 0.88 | 0.41 | 0.38 | 0.06 | 0.03 | 0.01 | 0.005 |
Most of the dissolved elements in the sea are found in ionic form constituting ionic sea salts. Majority of ionic sea salts result from the following compounds.
Sodium chloride‐NaCl, Magnesium Chloride‐MgCl2, Potassium Sulphate‐MgSO4, Calcium Sulphate‐ CaSO4, Potassium Sulphate‐K2SO4, Magnesium bromide‐MgBr2, & Potassium Chloride‐KCl.
The ocean is a dimensional body & so the distribution of physical properties like salinity, temperature 7 density is represented in a space by the help of coordinates of latitudes & longitudes with the additional factor of depth.
Salinity of Ocean water:
- Salinity is the total amount of solid material in a kilogram of sea water expressed in parts per thousand. The average salinity of seawater is 3.5% 7 is generally expressed as 35 parts per thousands.
The list of salts & there weight & percentage is given in the table.
| Salts | Weight (g) | % |
| Sodium Chloride | 27.213 | 77.8 |
| Magnesium Chloride | 3.809 | 10.9 |
| Magnesium Sulphate | 1.658 | 4.7 |
| Calcium Sulphate | 1.260 | 3.6 |
| Potassium Sulphate | 0.863 | 2.5 |
| Calcium Carbonate | 0.123 | 0.3 |
| Magnesium Bromide | 0.076 | 0.2 |
Change in salinity distribution
- Salinity changes due to winds resulting from differing in atmospheric pressure. The strong wind blowing throughout the year carry much of the warm & saline water from the western shore of the land in the
- lower middle latitudes & from the eastern shore in the higher latitudes resulting in changes in salinity distribution. The variations in salinity are according to the nature of the atmosphere i.e. the difference between precipitation 7 evaporation.
Distribution of Sea water | UPSC – IAS
Horizontal distribution
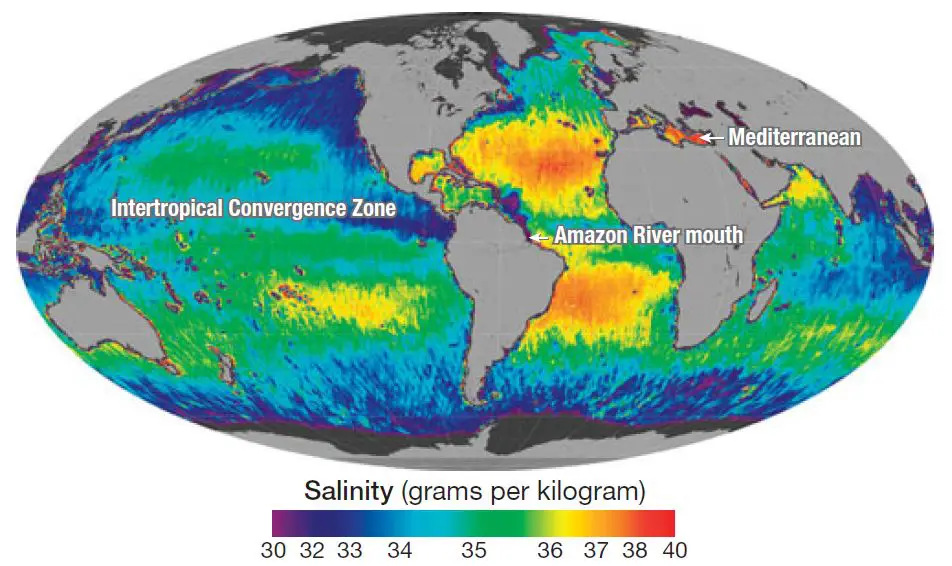
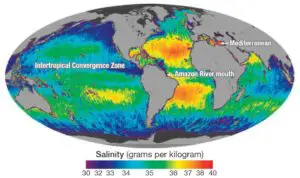
- Salinity is primarily controlled by latitude, & consequently decreases from the equator towards the pole. It is not maximum at the equator due to excess of rain over evaporation but the region of 20‐40 degree N records the highest average salinity of 36 % as a result of higher evaporation. In the southern hemisphere between 10‐30 degree S, 36% salinity is found. After obtaining maximum in the lower middle latitude, it again decreases between 40‐60 degree N & S & is 31% in northern hemisphere & 33% in southern hemisphere. Still lesser salinity is found in the polar areas due to the melting of ice. However, for the whole of northern hemisphere the average salinity observed is 34% 7 in southern hemisphere it is 35%. The different in these 2 is attributed to the abundance of ocean water in the Southern Hemisphere.
Vertical Distribution
- Salinity in the ocean decreases or increases in bottom according to the nature of the water mass. Those who attribute salinity to the atmospheric reaction conclude that due to the greater depth & less radius of influence of the greater depth & less radius of influence of the atmosphere salinity decreases at the bottom. This variation in salinity shows large difference with the latitude unless there is an occurrence of some cold or warm water mass which may result in drastic changes. At the southern boundary of the Atlantic salinity is 33.0, but; at 200 fathoms it reaches up to 34% increases to 34.50 still deeper at the bottom. Just at the equator the surface salinity of 34% increases with the depth to 35% due to greater mixture of fresh water at the surface.
- The saltiest water occurs in the Red Sea & the Persian Gulf where rates of evaporation are very high of the major oceans the North Atlantic is the saltiest its salinity averages about 37.9%.
- Generally it can be said that in high latitude salinity increases with depth due to dense water found at the bottom. Whereas in the middle latitude salinity increases with the depth up to 200 bottoms & then it starts decreasing. At equator due to a mixture of fresh water by rainfall surface salinity is low; just below this greater salinity is found which again decreases at the bottom due to the presence of cold water.
Density of Ocean water | UPSC – IAS
- The density of any substances is the mass per unit volume stated in grams per cubic centimeter. Commonly the word density is used for specific gravity which is the ratio of the density to that of distilled water at a given temperature & under atmospheric pressure.
- Pure water has maximum density of one unit at temperature 4 degree Celsius, whereas for the sea it changes according to the salinity content.
- Density of pure water depends upon temperature & pressure only.
- Whereas that of sea water depends upon temperature, pressure 7 salinity.
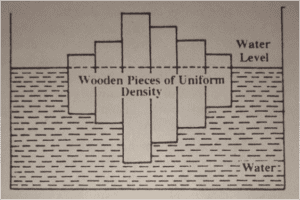
- This diagram shows the role of salinity on changes in density & freezing point.
- From the figure clear that pure clear that pure water freezes at 0 degree Celsius & has maximum density at 4 degree Celsius. The freezing point of ocean water decreases with increasing salinity so also the temperature of maximum density of.
Distribution of density of sea water | UPSC – IAS
- In general there is a latitudinal difference in the density distribution, depending on the character of the water changing from equator to the poles. The density of the upper layer commonly increases from the tropics towards the poles.
- The nature of the comparatively dense water in to sink down below the lighter water when 2 water masses having different density more dense water sinks down & then spreads out from the place where the similar density is found. It is observed that in the middle latitude, denser water sinks at lesser depths than the water that sinks at convergence in higher latitudes.
Vertical Distribution
The vertical distribution of density would reveal that generally at surface water of low density are found which increase in density towards the bottom. It is so because any amount of water which finds itself among less dense water would sink automatically below the surface up to that depth where water of similar density is found. Hence at places of convergences dense water mass sinks below the lighter one & forms bottom water. Nevertheless contrary to the surface current from the equator towards the pole there is bottom 7 this brings denser water under the surface of the sea.
Temperature of Ocean water | UPSC – IAS
- The study of temperatures of oceans is the subject matter of massive meteorology which deals with that portion of the atmosphere which overspreads the great water masses.
- The temperature of the sea & its accurate measurements has been one of the chief tasks of oceanographs, as it helps in the determination of the movements of large masses of ocean water.
There are various process of heating the ocean water
- By absorption of radiation from the sun
- Convection of heat through the ocean bottom from the interior of the earth.
- Transformation of kinetic energy into heat.
- Heating due to chemical processes.
- Convection of sensible heat from the atmosphere
- And condensations of water vapour.
Distribution of Temperature
The temperature & its distribution are determined by the following factors.
- The intensity & daily duration of solar radiated energy received.
- The depletion of this energy in this atmosphere by reflection, scattering & absorption.
- The albedo of the surface & its changes according to the angle of rays.
- Heat balance.
- Heat transfer through evaporation condensation.
- Important physical characteristics of the surface.
Ex – If the salinity of the ocean is greater the boiling point is raised & hence the temperature is high. Similarly if the density is lower than the temperature would be higher vice versa. But a combination of high temperature the amount of evaporation also determines the temperature.
Surface temperature of Ocean water | UPSC – IAS
A number of features which regard to the surface temperature of the ocean in relation to the latitudes are clearly brought out when we study the data given in the table below. The temperature decreases with the increasing distance from the equator. The decrease is about ½ per latitude though in actual observation it is slightly different. As a general rule the temperature decreases as the latitude increases but in all the oceans the higher values of the surface temperature are found to the north of equator.
| North | Atlantic | Indian | Pacific | Southern | Atlantic | Indian | Pacific | |
| latitude | Ocean | Ocean | Ocean | latitude in | Ocean | Ocean | Ocean | |
| in degree | degree | |||||||
| 70 | 60‐ | 5.60 | ‐ | ‐ | 70 – 60 | ‐1.30 | ‐1.50 | ‐1`.30 |
| 60 | 50‐ | 8.66 | ‐ | 5.74 | 60 – 50 | 1.76 | 1.63 | 5.00 |
| 50 | 40‐ | 13.16 | ‐ | 9.99 | 50 – 40 | 8.68 | 8.67 | 11.16 |
| 40 | 30‐ | 20.40 | ‐ | 18.62 | 40 – 30 | 16.90 | 17.00 | 16.98 |
| 30 | 20‐ | 24.16 | 26.14 | 23.38 | 30 – 20 | 21.20 | 22.53 | 21.53 |
| 10 | 0‐ | 25.81 | 27.23 | 26.42 | 20 – 10 | 23.16 | 25.85 | 25.11 |
| 26.66 | 27.85 | 27.20 | 10 0‐ | 25.18 | 27.41 | 26.01 | ||
Average surface temperature of the oceans between parallels of latitudes [temperature in degree centigrade].
Temperature beneath the surface
The sun rays have no direct effect below 600 feet & in spite of movement of water a vast bulk of sea is relatively cold. In general the complete stratification may appear in sea as lighter water of high salinity is found above the dense cold bottom water.
The following facts are marked as the characteristics of the vertical distribution of temperature of the sea.
- Firstly in spite of a general decrease of temperature towards the bottom the rate of fall is not equal at all depths up to 2000m the fall is rapid whereas it is almost stagnant below it.
- Secondly the surface temperature decreases with the increasing latitudes. Whereas the bottom temperature remains almost the same thus the rate of fall at equator is greater than at poles.
- The surface temperature & its decrease may be found to be influenced by the factor of upwelling of bottom water.
- In equatorial region an almost reversal of average conditions is found. At the surface the temperature & salinity is slightly lower due to abundant rainfall but just below it a layer exhibits high temperature & high salinity. Further at greater depth the usual decrease is found.
- Higher temperature is found at the bottom is due to insulation, anticyclonic circulation of currents around it & lesser mixing of cold waters.
Conclusion | UPSC – IAS
- In ocean the distribution of physical properties like salinity, density & temperature is represented with the help of coordinates of latitudes & longitudes with the additional factor of depths.
- Variations in salinity are according to the atmosphere i.e. the difference between precipitation and evaporation.
- Density of seawater depends upon the temperature, pressure and salinity.
- If the salinity is greater than the boiling point is raised & hence the temperature is high & also combination of high salinity & high density also produces high temperature.
- Temperature, density & salinity of sea water are inter‐related with each other.